Perhaps nothing captures the imagination of scientists and the general public alike more than the potential of stem cell therapies. These therapies direct pluripotent cells (i.e. cells that can become any cell in the body) to replenish cells that have been damaged or depleted by disease processes. Think diabetes, Parkinson’s Disease, ALS and even spinal cord injury. While the reality of stem cell therapies hasn’t always lived up to our expectations, the field of stem cell therapy research is still in its infancy and I think we have only begun to see the power of these therapies. One of the areas where stem cell therapies are starting to gain traction is in the treatment of DDD.
In our last post we discussed how a concoction of growth factors and cytokines maintains the health of the structural components of the intervertebral discs (IVDs). (For simplicity’s sake we’ll refer to these structural components wholly as extracellular matrix, or ECM.) These growth factors and cytokines stimulate cells within the IVD to produce ECM. For reasons that are not yet fully understood the populations of these cells, and thus amounts of ECM they produce, are depleted over time. This is where we get a “Chicken or Egg” scenario: is degenerative disc disease (DDD) caused by the loss of these cells or is some other trigger of DDD (i.e. years of mechanical stress) the cause of the loss of these cells. I’m not sure that the answer to this question is know yet, but what is known is that these populations of cells are tremendously important to the health of the IVD. The goal of stem cell therapies for DDD is to replace or regenerate these populations of IVD cells with the goal of replenishing the ECM components that give the disc its form and function. Sounds simple, right?
It turns out that it’s actually quite complicated. The first obstacle to overcome is where to get the “starter” cells used to replenish the lost populations of IVD cells. Probably the most straightforward way to replenish these cells is to just find a source of mature IVD cells for harvest. Some studies have looked at harvesting cells from herniated pieces of disc material removed at surgery. The number of cells within the IVD is quite low (they make up only 1% of the total disc volume) so after the cells are harvested from the disc fragments they must be cultured and multiplied until millions of cells are present. The cells can then be transplanted back into the diseased disc. Animal studies have confirmed that this is a viable method of increasing the number of IVD cells present and that these cells can improve the health of the IVD (See Figure 1). In one human study, the EuroDisc trial, cells were cultured and multiplied to over 5 million cells and then transplanted back into diseased discs at 12-weeks (so another procedure was required.) Although no placebo was used and there certainly were flaws in the assessment of outcomes, an initial analysis of EuroDisc data in 2008 revealed a decrease in pain and increase in disc hydration (as measured with MRI) at 2-year follow-up in patients who underwent cell transplantation versus controls. A more thorough analysis of their data was due out a few years ago but to my knowledge hasn’t been released.
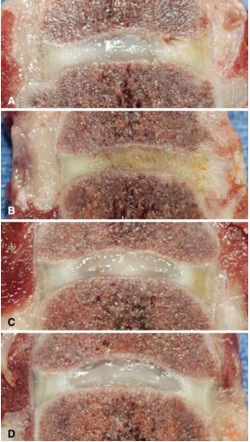
Figure 1. Specimens from dogs treated during animal phase of EuroDisc investigations. These are gross specimens analyzed 6-months after treatment. Image A is a control injured disc, note the dusky color, height loss and loss of distinction of the central nucleus pulposus. Image B is an injured disc injected with hyaluronic acid, looks even worse. Image C is an injured disc injected with adipose-derived stem cells while image D is a normal, uninjured disc. Note how the color, height and nucleus pulposus are preserved. (Source: Hohaus C, Ganey TM, Minkus Y, Meisel HJ: Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J 17 Suppl 4:492–503, 2008. Figure 6.)
Another way to grow a population of IVD cells is to start with true, pluripotent stem cells. These cells, capable of growing into a variety of tissue types, can be directed to become mature IVD cells. One main benefit of using stem cells as starter cells is that they can be easily obtained from the patient without the need for a spinal procedure to remove a piece of herniated disc to harvest cells from. The stem cells we’re discussing here aren’t embryonal stem cells which are harvested from an aborted fetus and thus are morally objectionable to some. Rather, these stem cells are mesenchymal stem cells (MSCs). The standard site of harvest of these MSCs is the bone marrow although other sites such as adipose tissue are becoming popular as well (so as a bonus you can get a liposuction during cell harvest!). Once MSCs are harvested they are cultured under specific conditions to become cells similar to those native to the IVD. These cells are then reimplanted into diseased discs to, it is hoped, regenerate the structural integrity of the disc. The feasibility of these methods has been confirmed in several animal studies. One pilot study done in humans (Orozco et al, 2011) has demonstrated not only feasibility and safety but also favorable clinical results in 10 patients with chronic back pain implanted with harvested MSCs.
In our third and final post of this series we’ll discuss some of the difficulties with MSC transplantation as well as some novel strategies that help overcome these difficulties.
Thanks for reading!
J. Alex Thomas, M.D.
Sources:
1. Chan SCW, Gantenbein-Ritter B: Intervertebral disc regeneration or repair with biomaterials and stem cell therapy–feasible or fiction? Swiss Med Wkly 142:w13598, 2012 Available: http://www.ncbi.nlm.nih.gov/pubmed/22653467. Accessed 17 July 2014
2. Hohaus C, Ganey TM, Minkus Y, Meisel HJ: Cell transplantation in lumbar spine disc degeneration disease. Eur Spine J 17 Suppl 4:492–503, 2008.
3. Orozco L, Soler R, Morera C, Alberca M, Sánchez A, García-Sancho J: Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation 92:822–8, 2011.
4. Sivakamasundari V, Lufkin T: Stemming the Degeneration: IVD Stem Cells and Stem Cell Regenerative Therapy for Degenerative Disc Disease. Adv Stem Cells:2013.
